
Introduction to SAS CLINICAL Training:
SAS CLINICAL Training- SAS Clinical is Statistical Analysis System Software. It was developed in North Carolina State University and it is mainly for the agricultural Purposes during 1966-1976. Mainly used for the Advanced Analytics, Business Intelligence, Data Management and Predictive Analytics. During 1980-1990 it is developed by adding statistical procedures, components and also introduction of JMP. SAS Clinical Corporate Training is a variety of software tools and packages available across the world and among these SAS CLINICAL is classified and data management tool is also a statistical tool.
Prerequisites for SAS Clinical Training:
To learn SAS Clinical course at our IdesTrainings the candidate should have a basic knowledge on
- Testing
SAS Clinical Corporate Training Course Details:
- Course Name: Clinical SAS Programming Corporate Training
- Mode Of Training: We provide both Corporate Training and Classroom Training.
- Duration: 30 Hrs (Can be customized as per the requirement)
- Materials: Yes we provide materials
- Fees: After the registration with IdesTrainings one of agents will assist you.
- Basic Requirement: Good Internet Speed and Headset
- Timings: According to the student flexibility
- Experience of trainer: 10+ years of experience
- Backup sessions: Yes we provide backup sessions
- Batch Type: We provide all batches like regular, weekends and Fast Track
CLINICAL SAS PROGRAMMING CORPORATE TRAINING COURSE CONTENT :
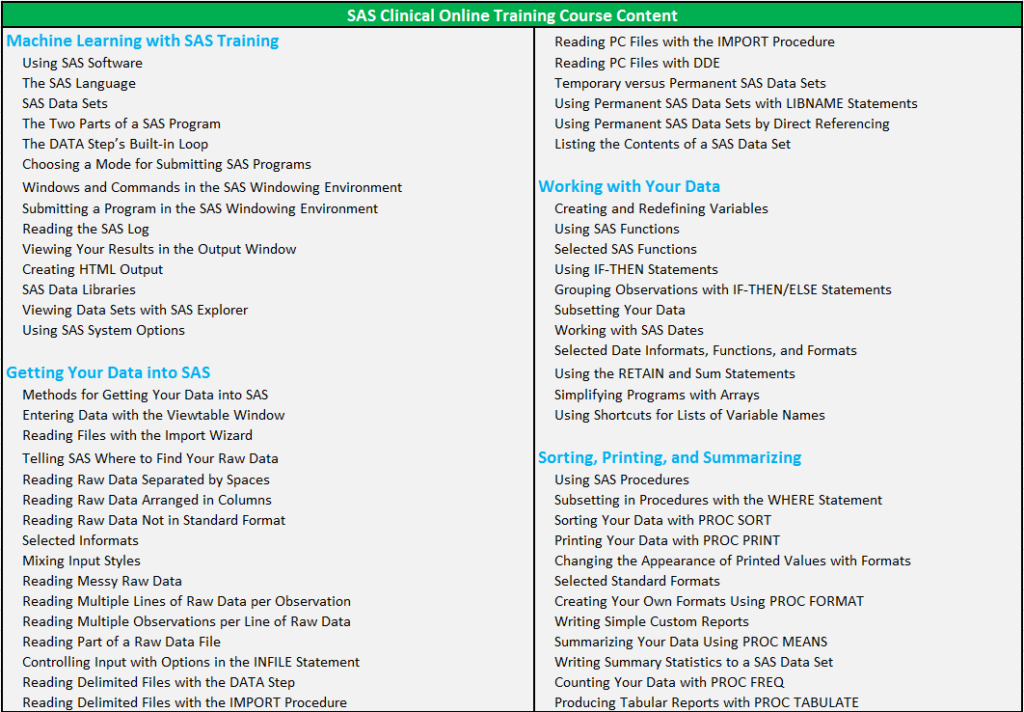
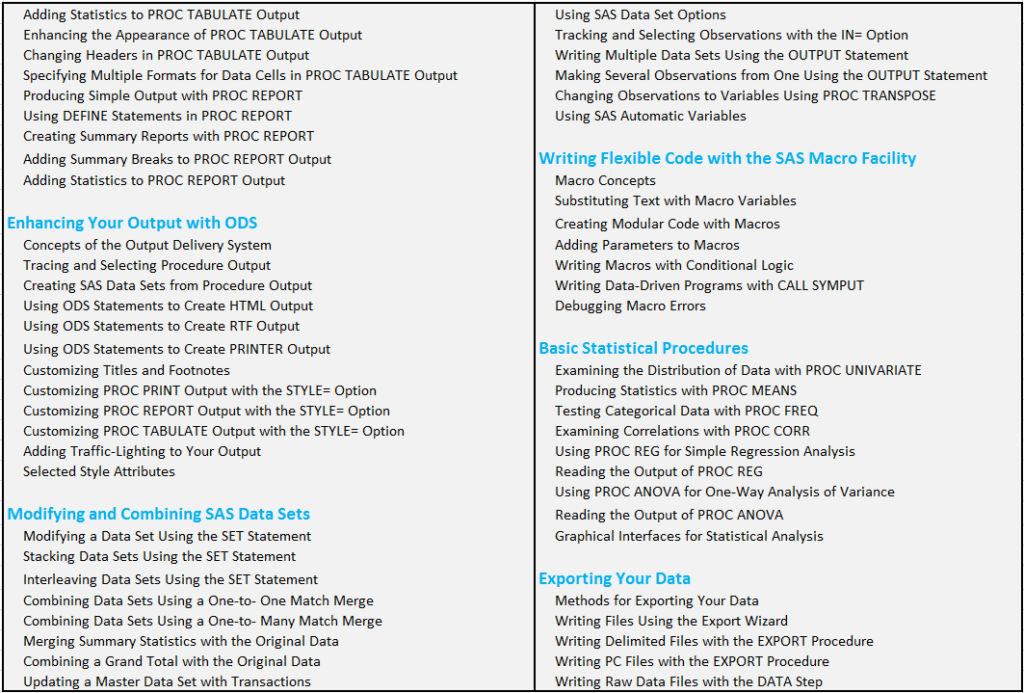
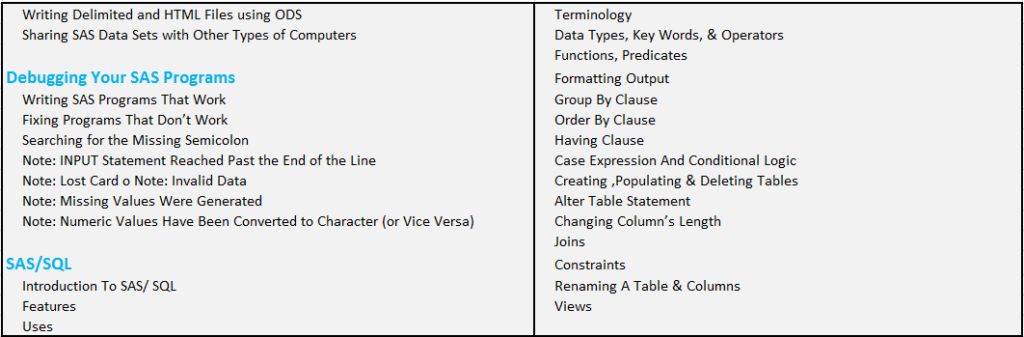
Overview of SAS Clinical Training:
What is SAS Competitors and Why is SAS Popular?
- SAS is used for the data management and also for Statistical analysis, so data management or data warehouse is nothing but it is used for reporting and ETL process, that is extract transform unload if you take Cognos and Tableau these are the reporting tools but they can’t perform the ETL are Statistical Analysis.
- Similarly Data Stage and Informatica they can naturally perform ETL process but neither reporting, not the statistical analysis, there are many other many statistical packages available like SPSS, Quantum, Statistica and R.
- so these can perform only statistical analysis but they not using reporting and ETL task. But SAS is the one which can perform all these 3 tasks that is nothing but ETL and Statistical analysis. That is the reason why SAS is becoming one outstanding analytical across the globe.
What is SAS Role in various Industries?
- It is used in various industries like Healthcare and pharmacy, Financial Service, Retail, and Telecom sector also it is used.
- Now see how SAS Clinical Training is useful to each industry if you take clinical sector SAS is mainly used in clinical crimes to perform Statistical analysis like calculating descriptive statistics like mean, standard deviation, medium values like that.
- Let’s take a small example here in clinical trials so many subjects are involved, so for each subject, several visits will be scheduled to take their values, for Bp or some time to take blood samples like this.
- So there are many visits’ finally we will take one value either that is an average or minimum value or the maximum value depending on the requirement, Clinical SAS Programming Training is easy to deal with large data SAS is being used.
Uses of the SAS Clinical Training:
Enables programmers:
- SAS is used for data warehousing ETL, it can perform reporting ETL process.
- It’s used for Data Cleaning; Data cleaning is nothing but any duplicate records are there we can delete the duplicate records using SAS.
- SAS Clinical Training is used for Analysis; we can calculate some Descriptive statistics like mean, average, standard deviation like that.
- And also using the SAS we can make Better Decisions
- SAS Clinical Training is used to Generate different kinds of reports like table listing configure and graphs
- SAS Clinical Training is used to Generate output in various formats like CSV, PDF, XLS, RTF any format we can generate output using SAS.
Could process – SAS Clinical Training:
- SAS Clinical Training can able to process Unlimited rows and columns
- It has many Built-in statistical functions for performing analysis
- It contains several numeric and characters functions for data manipulations
- And also interacts with multiple databases and operating systems
Clinical Trial Process:
- A SAS Clinical training trial or clinical research is nothing but a study conducted on a new drug before releasing that into a market, To start a clinical trial what are the required things initially, first, there should be a Sponsor and with him, he should have some new drug or new medicine anything.
- If he wants to release the new drug into the market he has to go to the FBA and get to an approval from FBA, as a having on new drug he cannot directly releasing into the market, when he approaches FBA, FBA will equation him why should I approve your drug, think that his new drug cure for the deck, then FBA will question him already market in so many drugs why should I again approve your drug.
- Then sponsor has to prove FBA that his drug is more efficient than the already existed drug. Think suppose already existed one curing the-deck in 5 hrs old one, but this new drug has to cure within 5 hrs or it should take 5 hrs then only FBA will approve. If it is taking more than 5 hrs then there is no use to releasing into the market. So sponsor has to prove his drug efficacy that it is more efficient than the already existing drug and also it should not contain side effects.
- Only acceptable side effects should be there, so for that he has to do some trail research process he has to conduct some study, they required some set of people will call them a subject, as it is new drug we don’t and whether it will give side effects or not, if it is giving again who will treat them. In general, if we are getting any abnormality decision to whom will go, will go to the doctors.
So he required some doctors also will call the investigators. Like this, some set of people are required. Let see step by step
Approved Protocol of SAS Clinical Training:
- Approved Protocol is nothing but an outline of a clinical trial. In general, protocol means nothing but a plan. Here also it should have one plan, in that protocol in this document sponsor has mentioned each and every detail about the clinical trial.
- Like how many days it going to happen, where it is going to happen in which hospital, whether the hospitals having all the laboratory equipment or not, on what kind of drug is going to do that research, what kind of subject his going to recruit about investigators about what kind of reports SAS programmers have to generate and everything should be mentioned in this.
- Once he prepared his protocol he has to send that to the FBA, FBA will check whether his plan is everything correct or not and then it will approve his protocol, once he gets his approval from the FBA then only he has to start the clinical trial process. He should not start whenever he wants. To start a clinical trial also he require should approve an FBA approval
Investigator selection of SAS Clinical Training:
Investigator selection based on his drug also it is mandatory right based on specialty the drug is about the unique drug.
SAS clinical administration:
- On one side the business needs are centred around less execution time, high edges, divided undertakings and the conveyance of top notch yield with insignificant oversight.
- On the opposite side, because of the expanded interest for talented assets, the needs of the software engineers have taken an alternate move toward enhancing presentation, unsustainable pay expansion because of numerous chances and by and large exclusive standards around vocation movement.
- In the event that the business needs won’t coordinate with developers need, or the other way around, at that point there is the likelihood that the present year on year development may begin to slow or even go into decrease.
- So as to break down the condition, a study was made utilizing on the web review entryway overview monkey and surveys were sent to directors and software engineers in the clinical SAS programming space.
Necessities:
- This is a positive pattern as the necessities are obviously deciphered and coordinating with the needs of the business. The following three bars show the delicate abilities where the administration evaluated desires are somewhat over the software engineer’s apparent regions to improve.
- This demonstrates a requirement for extra strides, to be taken by the administration, to guide their software engineers in sharpening their delicate aptitudes; this is particularly valid for senior developers, who are very nearly overseeing ventures and explicitly in zones of resourcing, coordinated effort, time the executives, clear correspondences, desires the board and partner the executives.
- The business standard reaches ought not to be connected with the pay rates that individuals get while moving onto a new position, as that pay will be higher as organizations utilize this to draw in ability.
- Investigation Dataset Models (ADaM). The CDISC ADaM group characterizes informational collection definition direction for the examination information structures. These informational indexes are intended for making measurable synopses and examination.
Operational Data Model:
- Operational Data Model (ODM). The ODM is an amazing XML-based information model that takes into account XML-based transmission of any information engaged with the lead of clinical preliminaries.
- SAS has offered help for bringing in and trading ODM records by means of the CDISC system and the XML LIBNAME motor. Case Report Tabulation Data Definition Specification (Define.xml).
- xml is the up and coming swap for the information definition record (define.pdf) sent to the FDA with electronic entries. Define.xml depends on the CDISC ODM model and is expected to give a machine-meaningful adaptation of define.pdf. Since define.xml is machine meaningful, the metadata about the accommodation informational indexes can be effectively perused by PC applications. This enables the FDA to work all the more effectively with the information submitted to it.
- Data Technology The data innovation (IT) bunch has changing duties, contingent upon the size of your association. IT is normally liable for PC frameworks foundation, upkeep, and general PC help work area support. The IT gathering may likewise play out some degree of programming advancement. In little to medium size associations IT might just make application program interfaces (APIs) between off-the-rack frameworks, while everywhere associations IT might be answerable for full programming applications design and advancement.
- You have to work with the IT office inside your association just as with outside supporters and merchants. Inside, you may work with IT for SAS arrangement the board and establishment capability, encryption innovations, and work area distributing or report circulation concerns. The most well-known explanation behind you to work with outside IT staff is as a rule as to data trade advances, for example, FTP and encryption apparatuses.
- The code support issue surfaces when you understand that you have to transform one of those SAS librefs. At that point you need to alter numerous SAS projects to make this basic change.
- An option in contrast to having those three SAS librefs wherever is to have them in a solitary area. The SAS large scale office gives two basic approaches to do this.
- The most ideal approach to ensure yourself against filthy clinical preliminary information is to utilize great protective programming methods.
- As it were, you ought to compose SAS code that records for every single imaginable datum stages. Envision you have a SAS informational collection that contains unfriendly occasion information for patients in a preliminary.
- Accept that the informational index has just three fields: the subject ID (subjectid), a “yes or no” field depicting whether the subject had an antagonistic occasion or not (aeyn), and the content portrayal of the unfavorable occasion (aetext).
- To separate information for the patients who had an unfriendly occasion, you may set up a SAS informational index as in the accompanying system.
SAS Macros:
- Use SAS Macros sensibly The SAS full scale language is an exceptionally amazing asset. With SAS macros you can compose dynamic SAS applications that are fundamentally SAS programs that compose different SAS programs.
- Sadly, with such incredible power comes the potential for extraordinary maltreatment.
- The SAS full scale language can be mishandled when it is utilized to such a degree, that a SAS program gets mixed up. A SAS large scale can become garbled when it is excessively thick with full scale summons, is ineffectively recorded, or includes too many settled full scale calls. For example, analyze the accompanying SAS code.
Patient recruitment of SAS Clinical Training:
- Patient recruitment they recruit healthy people and unhealthily people if the drug is to test about side effects are how the drug is working.
- Some side effects may get because of that cancer also, they recruit only cancer patients then according to study that study for the drug there are more side effects which are not true.
Data entry & validations to SAS Clinical Training:
- Data entry & validations in this team data entry persons and also data managers will enter the data they enter on paper and CRF.
- Again this case report form is different type one is paper CRF and another one is electronic CRF, nowadays we are not using paper CRFs, we may not say that each CRF will contain only one page or two pages, it depends on the study.
- If it is containing 50 pages those many patients how many pages it will take and they should not miss every single page also, the without missing single page they have sent it to FBA.
- So directly data entry persons will enter there and then they will convert into database. eCRF is looks like a paper but the format will be the same but in our system says the software will be installed, so directly data entry persons will enter there and then they will convert into the database.
- Nowadays they directly enter into a database that is called electronic data capture, it can take as this is database it has a large number of data. If you enter more data into your excel it will get hang it won’t open soon.
- But EDC it can take a large number of data, and also we have to maintain this data confidentially that security is very important. So using this EDC we can secure or data and it is very safe also.it can be accessible anywhere in the world and they having sponsor website details and internet connection they can log in anywhere.
Data managers of SAS Clinical Training:
Data managers this manager they will validate the data, whatever the data entered by data entry persons they will validate this data, why because they may not enter data correctly, so this data manager they will do validation by Edit checks.
Statistician (SAP) of SAS Clinical Training:
The statistician will have own document called SAP means statistician analysis plan, so in this plan based on the protocol, everything will be mention what kind of reports SAS programmer has to developed. So SAS programmers to generate different kinds of reports.
The medical writing team of SAS Clinical Training:
Medical writing team this medical writing person they will write a report, and again they submit to a statistician. Finally, this statistician will create CSR (Clinical study report), this re will submit the FDA it depends on the sponsor requirement; he wants to get the approval he will submit to FBA.
IdesTrainings provides best and quality real-time corporate training for SAS Clinical Training with reasonable price. Idestrainings is well said to be a reliable global leader in delivering the highest quality training in SAS Clinical Training. We also provide classes are available for the individual as well as for corporate batches on demand. We provide many Corporate courses for more information.
Along with SAS Clinical Training learn SAS CDM Training:
SAS CDM stands for SAS Clinical Data Management. A clinical information the board framework or CDMS is an instrument utilized in clinical research to deal with the information of a clinical preliminary. The clinical preliminary information accumulated at the agent site for the situation report structure are put away in the CDMS. To decrease the chance of blunders because of human section, the frameworks utilize different intends to check the information.
You can get the detail knowledge on SAS CDM Training along with SAS Clinical Training at our IdesTrainings.
Learn SAS Training along with SAS Clinical Training:
SAS stands for Statistical Analysis System. This is one of the software system used for Data Analysis and writing the report. This software can be used in graphical interface and also in SAS Programming Language.
Importance of SAS software:
- The data can be accessed in any format along with SAS Tables, Microsoft Excel Tables and also with database files.
- We can add or remove the data so that we can manage the data as we like.
- We can assume the data with the help of statistical techniques.
- The data we have assumed can be prepared in a meaningful report so that we can share our details with others and this reports can be saved in different formats.
These are just the basics of SAS. You can get the detail knowledge on SAS Training along with SAS Clinical Training.
Conclusion to SAS Clinical Training:
SAS Clinical Training is one of the most demanding courses in the present market. Many organizations are looking for the candidates who are having good communication skills and also practical knowledge on SAS Clinical Course. They are ready to pay huge salaries for the wright candidates. This is the wright time for the candidates who want to build their career in the field of SAS Clinical. If you have any doubts regarding the training always feel free to contact us or you can also register with us so that one of our coordinator will contact you as soon as possible.

